Current Issue
The Relationship of Metabolic Effects in Obese Adolescents with Serum Hepcidin Levels and Iron Metabolism
Kübra Arslan1,*, Ayça Törel Ergür2, Leman Gülsan Yavuz3
1Ufuk University Faculty of Medicine, Pediatrics Clinic, Turkey
2Ufuk University Faculty of Medicine, Department of Pediatric Endocrinology, ANKARA, Turkey
3Ufuk University Faculty of Medicine, Department of Pediatric Hematology and Oncology, ANKARA, Turkey
*Corresponding author: Dr. Kübra ARSLAN, Ufuk University Faculty of Medicine, Pediatrics Clinic, Turkey, ORCID: 0000-0002-9478-1463; Phone No: +905331924511; E-mail: [email protected]
Received Date: March 29, 2024
Publication Date: August 20, 2024
Citation: Arslan K, et al. (2024). The Relationship of Metabolic Effects in Obese Adolescents with Serum Hepcidin Levels and Iron Metabolism. Obese. 2(1):6.
Copyright: Arslan K, et al. © (2024).
ABSTRACT
Iron deficiency and subsequently developing iron deficiency anemia are among the important health issues globally associated with nutrition. One of these problems is iron deficiency, which develops due to increased hepcidin due to inflammation in obesity. This condition, which develops secondary to obesity, has been known for a long time. However, there are few studies on the relationship of increased hepcidin with metabolic disorders in obesity. We aim to examine the relationship of hepcidin with insulin resistance, hepatosteatosis and dyslipidemia, which are metabolic disorders in obesity.
Interconnected inflammatory mechanisms are expected to contribute to the development of inflammation-induced anemia. In our study, we observed an increased likelihood of developing iron deficiency when insulin resistance is present. However, in cases of isolated dyslipidemia or hepatosteatosis, there was no significant difference in the risk of iron deficiency among obese individuals. This finding suggests that insulin resistance is the primary driver of iron deficiency and elevated hepcidin levels, which are key components of the inflammatory cascade.
The noteworthy finding of lower hepcidin levels in cases with concurrent insulin resistance, dyslipidemia, and hepatosteatosis suggests a potential decrease in hepcidin production due to both intense chronic inflammation and liver steatosis-related hepatocyte damage.
Our study contributes to the growing understanding of the complex relationships between obesity, insulin resistance, dyslipidemia, hepatosteatosis, and their impact on iron metabolism.
For a comprehensive understanding of the mechanisms at play and the development of targeted treatments, further collaborative research is necessary.
Keywords: Iron, Nutrition, Obesity, Pregnancy
INTRODUCTION
Iron deficiency (ID) and subsequently developing iron deficiency anemia (IDA) are among the important health issues globally associated with nutrition [1]. The World Health Organization (WHO) identifies children, adolescents, and pregnant women as the most vulnerable groups for ID and IDA. The first peak for IDA is observed during infancy and the preschool period, while the second peak occurs in the adolescent age group [2]. Obesity, another significant health issue related to nutrition, has been referred to as the 'pandemic of the era' in recent years. The increase in the prevalence of obesity has also highlighted clinical conditions that develop as a result of obesity [3,4].
In a study conducted by Wenzel and colleagues, it was observed for the first time that iron deficiency is more frequent in obese adolescents compared to their non-obese peers [5]. This finding has brought about the necessity to investigate the association between obesity and iron deficiency. Studies have shown that a polypeptide named 'Hepcidin,' which is elevated due to subclinical inflammation observed in obesity, also contributes to susceptibility to iron deficiency [6].
Hepcidin Metabolism
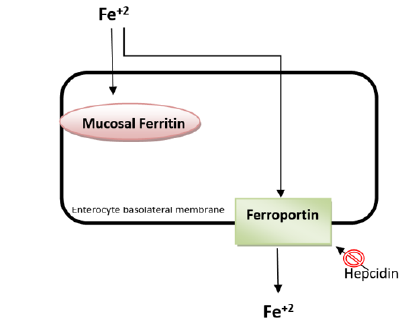
Hepcidin is the principal regulatory peptide controlling the amount of iron in circulation [7]. Structurally, it is an acute-phase reactant with antimicrobial properties. Synonyms for hepcidin include antimicrobial peptide (HAMP) and liver-expressed antimicrobial peptide-1 (LEAP-1) [8]. When serum iron levels increase and during inflammation, hepcidin expression also increases. The purpose of hepcidin is to prevent excessive iron burden and the damage caused by radical products that iron can generate within the body [9] (Figure-1).
Figure 1. Absorption of iron from enterocytes and the action site of hepcidin*.
*After iron is taken up by enterocytes, it is either stored as mucosal ferritin or released into the bloodstream for use. During release into the bloodstream, a transmembrane protein called 'ferroportin' is utilized on the basolateral membrane. If hepcidin levels in the blood rise for any reason, hepcidin binds to ferroportin, leading to its internalization and degradation. This results in the iron remaining inside the cell being directed towards the pathway of storage as mucosal ferritin [10].
The level of hepcidin in the blood is systemically controlled. Conditions such as blood loss, hypoxia, acute or chronic inflammatory processes, pregnancy, and situations that could lead to iron loss or hinder iron utilization, thereby causing iron deficiency, can alter hepcidin levels [11].
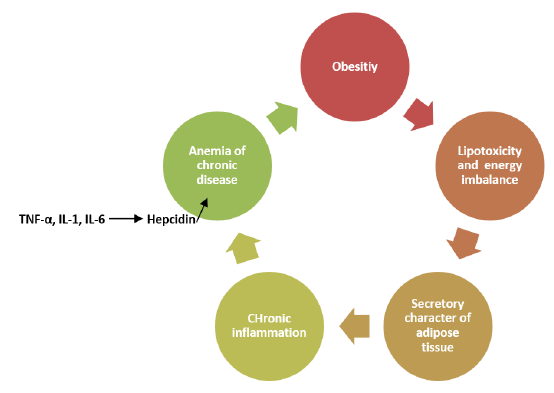
Recent studies have shown that adipose tissue functions as an endocrine organ. In addition to this role, adipose tissue produces numerous mediators with paracrine functions (such as adipokines, chemokines, cytokines, complement factors). These mediators are effective in adipose tissue and in the development of systemic inflammation [12,13]. As a result of this inflammation, a form of anemia similar to chronic disease anemia can be observed in obese individuals, independent of nutritional status. This perspective is gaining traction over time [6,14,15] (Figure-2). Furthermore, additional studies are needed to explore the relationship between glucose and lipid metabolism and hepcidin levels in obese cases. For all these reasons, the effects of hepcidin levels on obesity and iron metabolism have become an important research topic. Moreover, there is insufficient research on the relationship between metabolic status, hepsidin levels, and iron metabolism in obese children and adolescents. Therefore, our study aims to investigate the relationship between insulin resistance, dyslipidemia, hepatic steatosis, hepcidin levels, and iron parameters in obese adolescents.
Figure 2. Cascade of subclinical inflammation and chronic inflammation anemia in obesity [6,14,15].
MATERIAL AND METHODS
Our study included a total of 67 obese adolescents aged between 10 and 18 years, with pubertal stage 'Tanner Stage 2' and above. These cases were categorized based on significant metabolic components predicted to affect iron metabolism due to obesity, including thyroid functions, insulin resistance, dyslipidemia, and hepatic steatosis. Hepcidin levels and iron metabolism were compared between the groups.
Inclusion and exclusion criteria for the study are shown in Table-1.
Table 1. Inclusion and Exclusion Criteria for the Study
|
Inclusion Criteria for the Study |
|
|
Exclusion Criteria for the Study |
|
- Evaluation of Iron Metabolism in Cases
Among the cases constituting the study group, the individuals who will form the iron deficiency (ID) and iron deficiency anemia (IDA) group (Group 1) were determined by evaluating hemoglobin (Hb), hematocrit (Hct), ferritin, serum iron, total iron binding capacity (TIBC), and transferrin saturation (TS) in the complete blood count and in biochemical analysis. Although serum ferritin is an acute-phase reactant, it was considered for evaluation after excluding an acute inflammatory condition by simultaneously assessing CRP values. Obese adolescent cases without identified ID and/or IDA based on blood tests were categorized as Group 2.
The hematological parameters in the complete blood count of the cases were evaluated based on the updated values provided in Table-2.
Table 2. Normal hematological values in adolescents aged 10-18 years as per complete blood count [16].
|
Age |
Hemoglobin (g/dL) |
Hematocrit (%) |
MCV (fL) |
RDW (%) |
|
10-12 years |
||||
|
|
11.2-14.5 |
35-44 |
75-86 |
12.0-14.6 |
|
12-18 years |
||||
|
Girl |
11.4-14.7 |
36-46 |
80-96 |
11.9-14.6 |
|
Boy |
12.4-16.4 |
40-51 |
80-96 |
11.9-13.7 |
- Hepcidin Levels
Serum hepcidin levels were measured in all cases. Samples obtained from the patients were centrifuged at 4000 revolutions for 12 minutes to obtain serum samples, which were then stored at -200C. Hepcidin measurement was performed using the Human Hepcidin, HEPC ELISA Kit (BT Laboratory, Zhejiang, China) on the HEALES MB-530 device. The results were reported in pg/mL. Intra-assay and inter-assay %CV (Coefficient of Variation) values were given as 5.6% and <10%, respectively.
- Evaluation of Glucose and Lipid Homeostasis in Cases
The current glucose homeostasis of the cases was determined by evaluating fasting blood glucose, fasting insulin levels, and HOMA-IR (Homeostasis Model Assessment Insulin Resistance Index) levels, as well as oral glucose tolerance test. A HOMA-IR value above 2.5 in the prepubertal period and above 4.0 during puberty was considered indicative of insulin resistance [17] (Table 3).
Table 3.Evaluation of OGTT and HbA1c [18].
|
|
Normal |
Prediabetes |
Diabetes Mellitus |
|
Fasting Plasma Glucose |
<100 mg/dL |
100-125 mg/dL |
≥126 mg/dL |
|
2nd hours Plasma Glucose |
<140 mg/dL |
140-199 mg/dL |
≥200 mg/dL |
|
HbA1c |
<5.7% |
5.7-6.4% |
≥6.5% |
If a diagnosis of dyslipidemia was made, it was evaluated based on lipid parameters including total cholesterol, HDL, LDL, and triglyceride levels (Table 4).
Table 4. Normal values and dyslipidemia thresholds of serum lipid levels in childhood [19].
|
|
Normal values mg/dL |
Suspect values mg/dL |
High values mg/dL |
|
Total Cholesterol |
<170 |
170-199 |
>200 |
|
LDL |
<110 |
110-129 |
>130 |
|
HDL |
>45 |
40-45 |
<40 |
|
Triglyseride |
|||
|
0-9 years |
<75 |
75-99 |
>100 |
|
9-18 years |
<90 |
90-129 |
>130 |
- Evaluation of Thyroid Function in Cases
Due to the influence of thyroid metabolism on body fat composition [20], a thyroid panel was performed, including fT4 (free thyroxine), TSH (thyroid stimulating hormone), anti-thyroglobulin (TG), anti-thyroid peroxidase (TPO) antibodies, urinary iodine, and thyroid ultrasonography.
- Subclinical hypothyroidism was diagnosed based on a serum TSH level above normal but within the normal range of free T4.
- Overt hypothyroidism was defined as a low serum free T4 level with TSH >10mU/L.
- Autoimmune thyroiditis diagnosis was based on elevated thyroid autoantibodies and a heterogeneous appearance of the gland on thyroid ultrasonography (Anti-TG >30 U/ml, Anti-TPO >20 U/ml).
Euthyroid cases were those with serum TSH and fT4 levels within normal limits according to age and gender and had a normal gland volume on thyroid ultrasonography [21].
Statistical Analysis
Statistical analyses in this study were performed using SPSS 22 software package. The normal distribution of the data was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests and histogram plots. If the variables were normally distributed, parametric tests were used; otherwise, nonparametric tests were employed, and p<0.05 was considered statistically significant. The combined effect of numerical and categorical independent variables on the categorical dependent variable of iron deficiency/iron deficiency anemia status was analyzed using the 'Binary Logistic Regression' method. The combined effect of numerical and categorical independent variables on the numerical dependent variables of hemoglobin, hematocrit, serum iron, ferritin, transferrin saturation, and total iron binding capacity levels was analyzed using the General Linear Model Multivariate (GDM-MANCOVA) method. The combined effect of numerical and categorical independent variables on the numerical dependent variable of hepcidin was analyzed using the General Linear Model Univariate (GDM-ANCOVA) method.
Subgroup analyses:
Comparison of rates of categorical groups was performed using the Chi-Square test in 2x2 tables. Comparison of means of categorical groups was performed using the "Independent Samples T-test" or "Mann Whitney U" test. The relationship between numerical groups was analyzed using the "Pearson/Spearman Correlation" method. Descriptive statistics such as mean, standard deviation, or median for continuous variables and percentage frequencies for categorical variables were used.
RESULTS
- Demographic Characteristics of Included Cases, Anthropometric Evaluations, and Assessment of Compared Parameters
The demographic characteristics and anthropometric evaluations of the included cases are presented in Table-5. It can be observed that the demographic characteristics are evenly distributed among the groups. Additionally, it was noted that the body mass indexes of cases with iron deficiency/iron deficiency anemia (Group 1) were lower than those of the control group (Group 2), and this difference was statistically significant (p=0.014). Out of the cases, 34 were in the early pubertal stage, and 33 were in the late pubertal stage.
Table 5. Demographic characteristics and anthropometric evaluations of the cases.
|
|
Group 1 (n=28) |
Group 2 (n=39) |
P |
|
Sex (girl/boy) |
11/17 |
17/22 |
0.804 |
|
Age (years) |
13.00±2.08 |
13.11±2.43 |
0.835 |
|
Heigh (cm) |
161.49±10.63 |
159.73±11.49 |
0.517 |
|
Weigh (kg) |
80.86±20.40 |
72.68±16.56 |
0.075 |
|
BMI |
28.83±2.76 |
29.66±5.14 |
0.014 |
|
BMIP |
98.11±1.61 |
98.02±1.71 |
0.845 |
|
Early pubertal stage (P2-P3) |
15 |
19 |
0.806 |
|
Late pubertal stage (P4-P5) |
13 |
20 |
BMI: Body mass index, BMIP: Percentile of body mass index
When evaluating the iron parameters, CRP, and hepcidin values of the cases, it was observed that serum iron and transferrin saturation values were lower in Group 1 compared to Group 2, and this difference was statistically significant. Additionally, CRP and hepcidin values were significantly higher in Group 1 compared to Group 2. The comparison of iron and hepcidin parameters of the cases is presented in Table-6.
Table 6. Comparison of iron and hepcidin parameters of the cases.
|
Group 1 (n=28) |
Group 2 (n=39) |
p |
|
|
Hemoglobin (g/dL) |
13.74±1.33 |
15.11±1.15 |
0.769 |
|
Hematocrit (%) |
42.76±3.24 |
46.48±3.42 |
0.525 |
|
MCV (fL) |
79.33±4.26 |
83.50±3.51 |
0.249 |
|
RBC (M/µL) |
5.41±0.46 |
5.60±0.49 |
0.270 |
|
Ferritin (µg/L) |
31.82±21.50 |
46.28±37.82 |
0.064 |
|
Iron (µg/dL) |
47.64±12.79 |
94.79±24.86 |
<0.001 |
|
Iron binding capacity (µg/dL) |
347.75±57.05 |
256.67±36.26 |
0.068 |
|
Transferrin Saturation (%) |
12.05±2.50 |
27.86±6.84 |
<0.001 |
|
CRP (mg/dL) |
2.14±1.60 |
1.39±1.08 |
0.011 |
|
Hepcidin (ng/L) |
1310.54±1058.66 |
1155.632±709.03 |
0.023 |
Patients with overt hypothyroidism were not included in the study. However, the thyroid function tests of the cases were evaluated, revealing the presence of subclinical hypothyroidism. The assessment of the cases' thyroid function tests is presented in Table-7. Subclinical hypothyroidism was detected in 4 out of 67 subjects, and the primary cause identified for subclinical hypothyroidism was determined to be iodine deficiency. There were no cases of autoimmune thyroiditis among the subjects.
Table 7. Assessment of the cases' thyroid function tests.
|
|
Group 1 (n=28) |
Group 2 (n=39) |
p |
|
TSH |
2.52±1.30 |
2.07±0.79 |
0.004 |
|
Free T4 |
1.02±0.90 |
1.18±0.16 |
0.089 |
- Relationship Between Serum Hepcidin Levels and Endocrine Parameters / Hepatosteatosis in Cases
When considering the combined effect of independent numerical and categorical variables on the presence of iron deficiency/iron deficiency anemia, it was determined that insulin resistance had an effect on the status of ID/IDA.
Hemoglobin, hematocrit, serum iron, ferritin, transferrin saturation, and total iron-binding capacity levels, which could affect numerical dependent variables, were evaluated through multivariate analysis considering gender, hepcidin levels, insulin resistance, dyslipidemia, and hepatosteatosis. It was observed that gender, hepcidin levels, insulin resistance, dyslipidemia, and hepatosteatosis did not have direct significant effects on iron parameters.
When assessing the combined effect of numerical and categorical independent variables on the numerical dependent variable of hepcidin, it was found that hepcidin levels were influenced and statistically lower in cases with both insulin resistance, dyslipidemia, and hepatosteatosis. In cases with all three conditions (insulin resistance, dyslipidemia, and hepatosteatosis), the average hepcidin level was 853.245 pg/mL, while the average hepcidin level in other cases was 1196.038 pg/mL (p=0.03).
Table 8. Comparison of Hepcidin Levels and Iron Parameters in the Presence of Insulin Resistance, Dyslipidemia, or Hepatosteatosis
|
|
Hb(g/dL) (Mean±SD) |
Hct (%) (Mean±SD) |
Iron (µg/dL) (Mean±SD) |
IBC (µg/dL) (Mean±SD) |
TS% (Mean±SD) |
Ferritin (ng/mL) (Mean±SD) |
Hepcidin (pg/mL) (Mean±SD) |
|
Insulin Resistance |
|||||||
|
Yes |
14.62±1.64 |
45.30±4.52 |
67.20±29.04 |
323.10±57.36 |
18.60±10.36 |
31.40±27.40 |
1220.40±1065.96 |
|
No |
14.51±1.30 |
44.77±3.50 |
78.45±31.74 |
282.66±63.86 |
22.36±9.07 |
44.00±34.18 |
1220.36±782.50 |
|
p |
0.108 |
0.141 |
0.674 |
0.834 |
0.680 |
0.658 |
0.673 |
|
Dyslipidemia |
|||||||
|
Yes |
14.27±1.45 |
46.28±3.41 |
83.44±34.15 |
278.56±59.61 |
23.68±9.43 |
53.93±42.43 |
1250.94±868.33 |
|
No |
14.93±1.25 |
44.01±3.82 |
69.45±28.03 |
305.65±65.80 |
19.61±9.39 |
31.00±19.59 |
1199.73±878.65 |
|
p |
0.363 |
0.391 |
0.233 |
0.791 |
0.717 |
0.001 |
0.608 |
|
Hepatosteatosis |
|||||||
|
Yes |
14.56±1.60 |
45.18±4.18 |
73.59±30.05 |
302.13±69.24 |
21.45±10.29 |
45.75±40.13 |
1186.41±783.10 |
|
No |
14.52±1.20 |
44.70±3.48 |
76.46±32.54 |
287.97±59.68 |
21.03±8.97 |
35.20±23.29 |
1251.42±949.83 |
|
p |
0.050 |
0.270 |
0.609 |
0.931 |
0.522 |
0.047 |
0.357 |
Hb: Hemoglobin, Hct: Hematocrit, IBC: Iron binding capacity, TS%: Transferrin Saturation
When considering only the presence of insulin resistance in the cases, no statistically significant differences were observed in hematological values and iron parameters between cases with insulin resistance and those without it. Similarly, when cases were classified based solely on the presence of dyslipidemia, it was observed that cases with dyslipidemia had higher ferritin levels compared to those without dyslipidemia, and this difference was statistically significant (p=0.001). When cases were classified according to the presence of hepatosteatosis, it was determined that cases with hepatosteatosis had higher ferritin levels compared to cases without hepatosteatosis, and this difference was statistically significant (p=0.047). No significant differences in hepsidin levels were observed among the three groups.
Table 9. Rates of Insulin Resistance, Dyslipidemia, and Hepatosteatosis in Cases with Iron Deficiency/Iron Deficiency Anemia
|
ID/IDA |
Insulin Resistance |
Dyslipidemia |
Hepatosteatosis |
|
Yes (n=28) |
12/28 (42.85%) |
8/28 (28.57%) |
14/28 (50.00%) |
|
No (n=39) |
8/39 (20.51%) |
19/39 (48.71%) |
18/39 (46.15%) |
When the cases were divided into the iron deficiency/iron deficiency anemia group (n=28) and the control group (n=39), it was determined that insulin resistance and hepatosteatosis were proportionally higher in the ID/IDA group, while dyslipidemia was more prevalent in the control group.
DISCUSSION
Studies conducted over the past 60 years have drawn attention to chronic inflammation and the potential development of iron deficiency anemia (IDA) alongside the well-known comorbid conditions of obesity [3-5]. As a result, numerous studies have investigated the impact of obesity on iron metabolism in different age groups. However, research focusing on adolescents, who are highly susceptible to both obesity and IDA, remains limited.
In the context of insulin resistance, obesity is the primary etiopathology that comes to mind in contemporary discussions [22]. While it has been proposed that pathologies stemming from intracellular signaling pathways of the insulin receptor might contribute, the secretory properties of increased adipose tissue in obesity have also gained attention. Elevated levels of circulating free fatty acids and the secretion of molecules such as leptin, adiponectin, and resistin from adipose tissue likely contribute to the development of insulin resistance. Moreover, the abnormal expansion of adipose tissue and its increased activity in obesity lead to an inflammatory process, where inflammatory cytokines may also play a role in the development of insulin resistance [14,22]. Insulin resistance disrupts the functions of adipocytes within adipose tissue, resulting in structural changes. These altered adipocytes not only contribute to the development of dyslipidemia but also become structurally insulin-resistant [15,23]. This situation indicates that insulin resistance is a key factor underlying both dyslipidemia and hepatosteatosis.
Interconnected inflammatory mechanisms are expected to contribute to the development of inflammation-induced anemia. In our study, we observed an increased likelihood of developing iron deficiency when insulin resistance is present. However, in cases of isolated dyslipidemia or hepatosteatosis, there was no significant difference in the risk of iron deficiency among obese individuals. This finding suggests that insulin resistance is the primary driver of iron deficiency and elevated hepcidin levels, which are key components of the inflammatory cascade.
CONCLUSION
In conclusion, our study contributes to the growing understanding of the complex relationships between obesity, insulin resistance, dyslipidemia, hepatosteatosis, and their impact on iron metabolism. The identification of insulin resistance as a central player in these interactions emphasizes the need for further comprehensive investigations that consider the metabolic status, liver steatosis, and inflammatory parameters collectively.
Previous studies have demonstrated the involvement of inflammatory processes in obesity, insulin resistance, dyslipidemia, and hepatosteatosis, all of which are associated with elevated hepcidin levels and a predisposition to iron deficiency [15,23-25]. When evaluating the cases included in our study, the lack of significant differences in hepcidin levels between individuals with insulin resistance, dyslipidemia, or hepatosteatosis could potentially be attributed to the overall obesity status of our participants and the presence of similar levels of subclinical inflammation. However, the noteworthy finding of lower hepcidin levels in cases with concurrent insulin resistance, dyslipidemia, and hepatosteatosis suggests a potential decrease in hepcidin production due to both intense chronic inflammation and liver steatosis-related hepatocyte damage.
For a comprehensive understanding of the mechanisms at play and the development of targeted treatments, further collaborative research is necessary. This research should encompass the evaluation of metabolic statuses, liver steatosis levels, and inflammatory parameters in these subjects.
This work was supported by Ufuk University (LTP2022-01).
REFERENCES
- Camaschella C. (2015). Iron-deficiency anemia. N Engl J Med. 372(19):1832-1843.
- Report of the WHO Consultation on Iron Deficiency Anemia. (2001). Assessment, Prevention, and Control A guide for programme managers, Geneva.
- Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. (2015). Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 16(12):1081-1093.
- Report of the WHO Consultation on Obesity. (1997). Prevention and management of the global epidemic of obesity, Geneva. p. 3-5.
- Wenzel BJ, Stults HB, Mayer J. (1962). Hypoferraemia in obese adolescents. Lancet. 2(7251):327-328.
- Fraenkel PG. (2017). Anemia of Inflammation: A Review. Med Clin North Am. 101(2):285-296.
- Weizer-Stern O, Adamsky K, Margalit O, Ashur-Fabian O, Givol D, Amariglio N, et al. (2007). Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. Br J Haematol. 138(2):253-262.
- Stefanova D, Raychev A, Arezes J, Ruchala P, Gabayan V, Skurnik M, et al. (2017). Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood. 130(3):245-257.
- Camashella C. (2020). Regulation of iron balance: intestinal iron absorption. In: UpToDate [online]. Available at: www.UpToDateInc.com/card.
- Dasa F, Abera T. (2018). Factors Affecting Iron Absorption and Mitigation Mechanisms: A review. Int J Agric Sc Food Technol. 4:24-30.
- Steele TM, Frazer DM, Anderson GJ. (2005). Systemic regulation of intestinal iron absorption. IUBMB Life. 57(7):499-503.
- Cox AJ, West NP, Cripps AW. (2015). Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3(3):207-215.
- Ghosh S, Ashcraft K. (2013). An IL-6 link between obesity and cancer. Front Biosci (Elite Ed). 5(2):461-478.
- Ganz T, Nemeth E. (2011). Hepcidin and disorders of iron metabolism. Annu Rev Med. 62:347-360.
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 17(1):4-12.
- Powers JM, Sandoval C. (2022). Approach to the child with anemia. In: UpToDate [online]. Available at: www. UpToDate.Com.
- Valerio G, Licenziati MR, Iannuzzi A, Franzese A, Siani P, Riccardi G, Rubba P. (2006). Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutr Metab Cardiovasc Dis. 16(4):279-284.
- Type 2 diabetes in children and adolescents. (2000). American Diabetes Association. Diabetes Care. 23(3):381-389.
- Dai S, Yang Q, Yuan K, Loustalot F, Fang J, Daniels SR, Hong Y. (2014). Non-high-density lipoprotein cholesterol: distribution and prevalence of high serum levels in children and adolescents: United States National Health and Nutrition Examination Surveys, 2005-2010. J Pediatr. 164(2):247-253.
- Laurberg P, Knudsen N, Andersen S, Carlé A, Pedersen IB, Karmisholt J. (2012). Thyroid function and obesity. Eur Thyroid J. 1(3):159-167.
- Alpcan A, Törel Ergür A, Tursun S. (2017). Insidious danger in childhood era’s; subclinical hypothyroidism. Ortadogu Med J. 9(1):34-38.
- Mantzoros C. (2020). Insulin resistance: Definition and clinical spectrum. In: UpToDate [online]. Available at: www.UpToDate.com.
- Schmidt PJ. (2015). Regulation of iron metabolism by hepcidin under conditions of inflammation. J Biol Chem. 290(31):18975-18983.
- González-Domínguez Á, Visiedo-García FM, Domínguez-Riscart J, González-Domínguez R, Mateos RM, Lechuga-Sancho AM. (2020). Iron Metabolism in Obesity and Metabolic Syndrome. Int J Mol Sci. 21(15):5529.
- Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E. (2011). Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res. 158(6):369-384.
 Abstract
Abstract  PDF
PDF